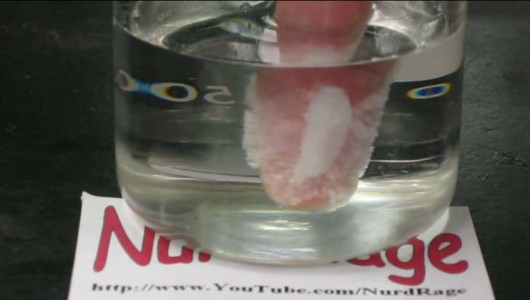
This fact may surprise you but it seems that “hot ice” is indeed a real stuff. This video demonstrates some special properties of this solution, that is, ability to liquefy and solidify in the matter of a few seconds. Discover what happens when you dip your hand in it!
The “hot ice”, sometimes called “sodium acetate trihydrate”, is a material that has the distinction of solidifying at room temperature and melting at 58°C. When cooled quickly, the “hot ice” changes its form from liquid to solid condition without crystallising. If an object like hand is introduced into the “hot ice” in the molten form, the liquid instantly crystallizes around it. In this video of NurdRage, the coating of sodium acetate on his hands quickly crystallizes within matter of seconds. Substance then obtained is stronger. It is described as warm, with the consistency of the ice cream.
[youtube]http://www.youtube.com/watch?v=7HDZI2rwyHg[/youtube]
The speed of the chemical reaction is impressive. It must be very special to have dipped the hand in the “hot ice”. As for you, would you be willing to experiment and plunge your hand into a pot of “hot ice”?