The very purpose of physics is to quantitatively describe and explain the physical world in as few terms as possible. This principle extends to units of measurement as well, which is why we usually find different units used in science actually defined in terms of more fundamental units. The watt, for example, is one joule of energy transferred per second of time. The joule, in turn, is defined in terms of three base units, the kilogram, the meter, and the second:
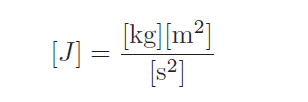
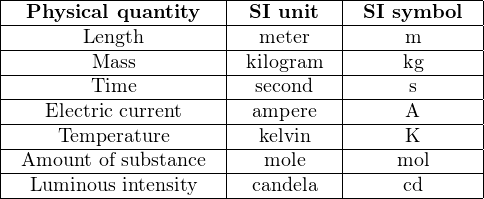
Within the metric system of measurements, an international standard exists for which units are considered fundamental and which are considered “derived” from the fundamental units. The modern standard is called SI, which stands for Système International. This standard recognizes seven fundamental, or base units, from which all others are derived4 :
An older standard existed for base units, in which the centimeter, gram, and second comprised the first three base units. This standard is referred to as the cgs system, in contrast to the SI system5 . You will still encounter some derived cgs units used in instrumentation, including the poise and the stokes (both used to express fluid viscosity). Then of course we have the British engineering system which uses such wonderful6 units as feet, pounds, and (thankfully) seconds. Despite the fact that the majority of the world uses the metric (SI) system for weights and measures, the British system is sometimes referred to as the Customary system.
2.7 Conservation Laws
The Law of Mass Conservation states that matter can neither be created nor destroyed. The Law of Energy Conservation states that energy can neither be created nor destroyed. However, both mass and energy may change forms, and even change into one another in the case of nuclear phenomena.
Conversion of mass into energy, or of energy into mass, is quantitatively described by Albert Einstein’s famous equation:
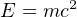
Where,
E = Energy (joules)
m = Mass (kilograms)
c = Speed of light (approximately 3 × 108 meters per second)
Conservation laws find practical context in many areas of science and life, but in the realm of process control we have the principles of mass balance and energy balance which are direct expressions of these Laws. “Mass balance” refers to the fact that the sum total of mass entering a process must equal the sum total of mass exiting the process, provided the process is in a steady-state condition (all variables remaining constant over time). To give a simple example of this, the mass flow rate of fluid entering a pipe must be equal to the mass flow rate of fluid exiting the pipe, provided the pipe is neither accumulating nor releasing mass within its internal volume. “Energy balance” is a parallel concept, stating that the sum total of energy entering a process must equal the sum total of energy exiting a process, provided a steady-state condition (no energy being stored or released from storage within the process).
